Chondrosarcoma of the femur: Extra-articular resection and endoprosthetic reconstruction (Adler Pantheum) plus lateral gastrocnemius flap
Overview
Subscribe to get full access to this operation and the extensive Bone & Soft Tissue Tumour Surgery Atlas.
Professional Guidelines Included
Learn the Chondrosarcoma of the femur: Extra-articular resection and endoprosthetic reconstruction (Adler Pantheum) plus lateral gastrocnemius flap surgical technique with step by step instructions on OrthOracle. Our e-learning platform contains high resolution images and a certified CME of the Chondrosarcoma of the femur: Extra-articular resection and endoprosthetic reconstruction (Adler Pantheum) plus lateral gastrocnemius flap surgical procedure.
Chondrosarcoma (CS) is the second most common primary bone tumour and the most common in adults. These range from low to high grade malignant cartilage tumours which may metastasise to the lungs. They are relatively insensitive to chemo- or radiotherapy meaning that surgery is the principal intervention. Five year survival ranges from 99% for low grade to 24% for dedifferentiated chondrosarcomas. The distal femur is the most common site of primary malignant bone tumours in all age groups followed by the proximal femur, proximal humerus and pelvis. Chondrosarcomas can arise de-novo, as in this case, and also arise in other pre-existing conditions such as hereditary multiple osteochondromas (diaphysial aclasis) and multiple enchondromatosis (Ollier disease and Maffucci syndrome).
Achieving adequate surgical margins to ensure the tumour is excised-bloc is the guiding oncological principle to avoid local recurrence; local recurrence is associated with metastasis and death.
The oncological principle is to widely resect the tumour with the biopsy tract in-situ with adequate surgical margins in all planes to minimise the risk of local recurrence and reconstruct the segmental osseous defect with endoprosthesis, allograft or autograft. Endoprosthetic replacement is most commonly used when en-bloc resection involves the hip, knee or glenohumeral joint as these reconstructions offer early weight-bearing and return to adequate function with acceptable complication rates. In patients in whom limb-salvage is impossible due to tumour encroachment on nerves, blood vessels and joints, amputation may regrettably be the optimal oncological treatment for their chondrosarcoma.
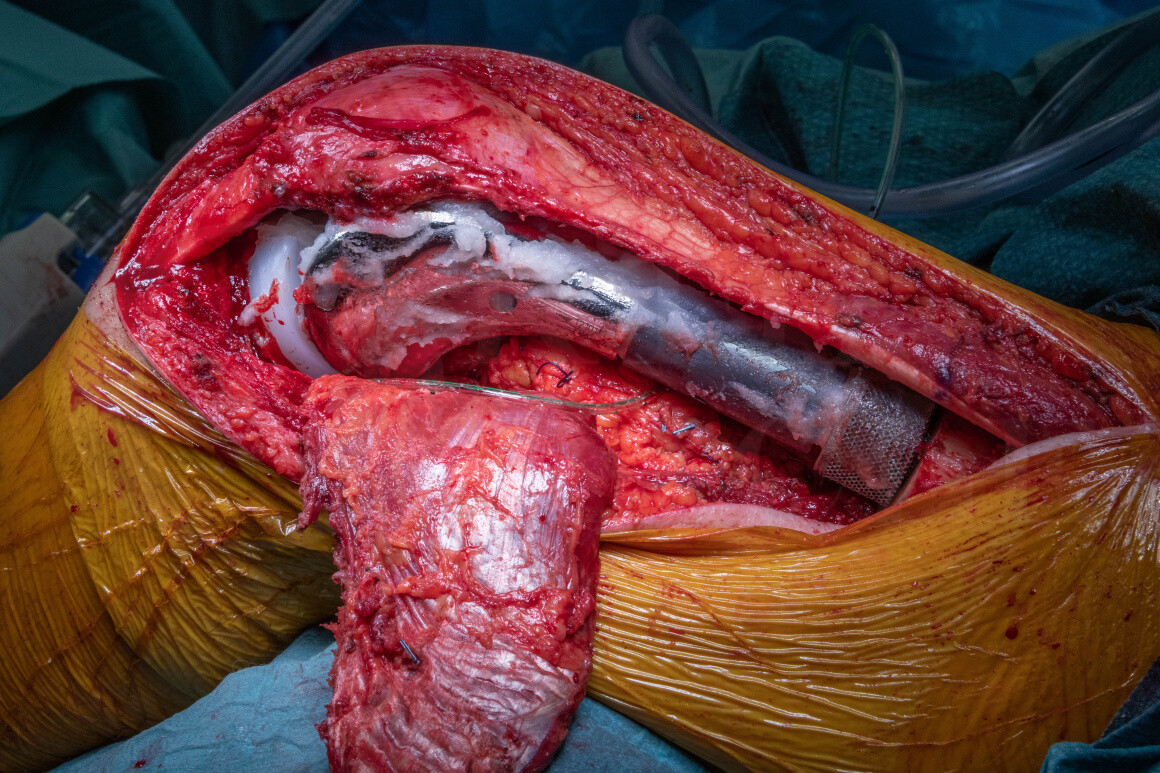
In some cases the tumour extension has involved the joint cavity of the knee which would lead or compromised surgical margins if the joint capsule were exposed peri-operatively. This may be clear on pre-operative imaging or be suspected due to subtle joint effusions or extension of pathological fractures into the joint radiologically. Consequently a demanding ‘extra-articular’ excision is undertaken to excise the distal femur and proximal tibial epiphysis en-bloc without performing an arthrotomy or exposing the tumour.
The Adler Pantheon limb-salvage system has been designed to offer the latest technology combined with simplicity of use. Key features include the additive manufactured bridging collar which has an integrated endosteal sleeve with a porous structure to allow osseointegration, Agluna silver coating to help prevent early infection, a unique cement pressuriser, the ability to change rotation of the prosthesis in any direction after cementation and a self-catering rotating polyethylene bearing to aid stability.
OrthOracle reader will find the following related operative techniques also of interest:
Forequarter amputation for chondrosarcoma
Hindquarter amputation with pedicled fillet flap for clear cell chondrosarcoma of the proximal femur
Proximal femoral endoprosthetic replacement (Stanmore METS, Stryker) for chondrosarcoma
Author: Jonathan Stevenson FRCS (Tr & Orth)
Institution: The Royal Orthopaedic Hospital, Birmingham, UK.
Clinicians should seek clarification on whether any implant demonstrated is licensed for use in their own country.
In the USA contact: fda.gov
In the UK contact: gov.uk
In the EU contact: ema.europa.eu



















