Femoral endoprosthesis: MUTARS intercalary EPR (Implantcast) for pathological fracture of spindle cell sarcoma of bone
Overview
Subscribe to get full access to this operation and the extensive Bone & Soft Tissue Tumour Surgery Atlas.
Learn the Femoral endoprosthesis: MUTARS intercalary EPR (Implantcast) for pathological fracture of spindle cell sarcoma of bone surgical technique with step by step instructions on OrthOracle. Our e-learning platform contains high resolution images and a certified CME of the Femoral endoprosthesis: MUTARS intercalary EPR (Implantcast) for pathological fracture of spindle cell sarcoma of bone surgical procedure.
Osteosarcoma, Ewing’s sarcoma and chondrosarcoma are the three most common primary malignant sarcomas of bone. There are a number of other primary malignant bone sarcomas which do not fit neatly into one of the above three types, and these are generally known as ‘spindle cell sarcomas of bone’. They are rare and include such entities as fibrosarcoma, malignant fibrous histiocytoma and leiomyosarcoma
The most commonly effected anatomical sites are the femur followed by the tibia, pelvis and humerus. The typical age for a spindle cell sarcoma of bone is 45 years and 25% of patients present with a pathological fracture.
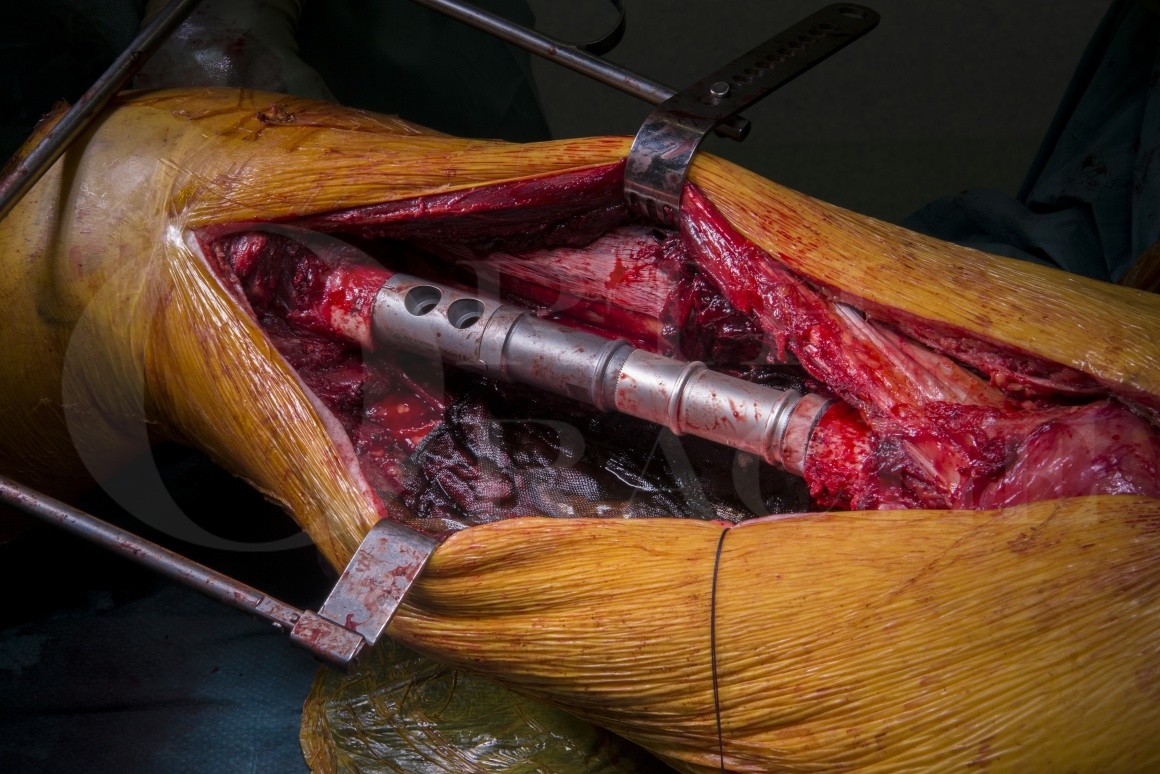
Endoprosthetic reconstruction of the femoral diaphysis, although scarcely reported in the literature, generally results in a reasonable functional outcome, allowing patients to bear weight and resume near normal function swiftly.
I use the Implantcast MUTARS diaphyseal endoprosthesis because (to my knowledge) it is the only silver coated, modular intercalary endoprosthesis on the market which also offers short and long reconstruction lengths (100 to 320mm), cemented or cement-less stems and if one of the implant stems loosens or fractures it can be converted to a proximal, or distal femoral endoprosthesis without explanting the other fixed stem.
Author: Jonathan Stevenson, FRCS (Tr & Orth).
Institution: Royal Orthopaedic Hospital, Birmingham
Clinicians should seek clarification on whether any implant demonstrated is licensed for use in their own country.
In the USA contact: fda.gov
In the UK contact: gov.uk
In the EU contact: ema.europa.eu
Online learning is only available to subscribers.



















