Knee arthroscopy and microfracture of osteochondral defect
Overview
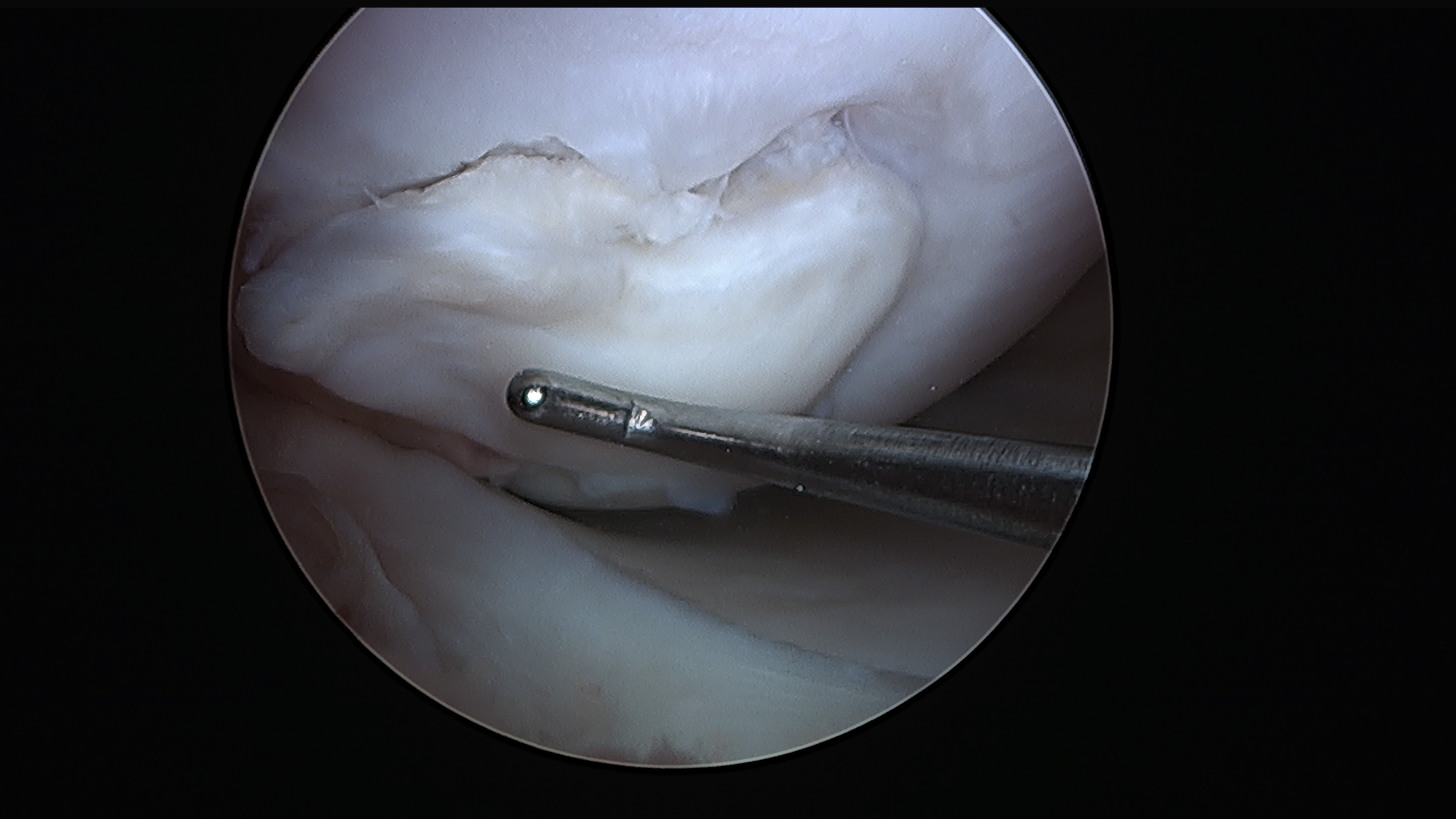
Subscribe to get full access to this operation and the extensive Knee Surgery Atlas.
Learn the Knee arthroscopy and microfracture of osteochondral defect surgical technique with step by step instructions on OrthOracle. Our e-learning platform contains high resolution images and a certified CME of the Knee arthroscopy and microfracture of osteochondral defect surgical procedure.
Microfracture is a form of cartilage repair that was popularised in the 1980s by Richard Steadman in Colorado, USA. It is not overly technically demanding and has shown good functional outcomes for small (<2cm2) lesions. Original data from the 1980s suggested its use for larger lesions (up to 4cm2). More recent evidence such as NICE guidance and alternative treatments, such as autologous chondrocyte implantation (ACI), have narrowed the indications of microfracture based on size.
It is a straightforward procedure to undertake in one sitting and worthwhile for appropriate sized lesions. However it must be appreciated that microfracture creates repair-type fibrocartilage rather than hyaline cartilage, which is inferior in terms of biomechanics and longevity.
Modern cartilage regeneration techniques are also available and garnering good evidence, especially for larger lesions. Autologous cartilage implantation (ACI) for example is a much more costly, 2-stage procedure. The size of the lesion therefore is critical in the decision making process as the results of ACI can be impaired by previous failed regenerative procedures including microfracture.
OrthOracle readers will also find the following instructional techniques of interest:
Stem cell harvest and transplant for knee osteochondral defect (Synergy Medical technologies)
Femoral trochlea chondral lesion: Chondrogide membrane(Geistlich pharma) for chondral regeneration.
Arthroscopic medial menisectomy and chondroplasy of knee
Author: Author :Mr Andrew Pearse FRCS (Tr & Orth)
Institution: Institution: The Worcestershire Acute Hospitals NHS Trust, UK.
Clinicians should seek clarification on whether any implant demonstrated is licensed for use in their own country.
In the USA contact: fda.gov
In the UK contact: gov.uk
In the EU contact: ema.europa.eu
Online learning is only available to subscribers.



















