Pilon fracture: C-type fixed using Smith and Nephew EVOS small fragment system
Overview
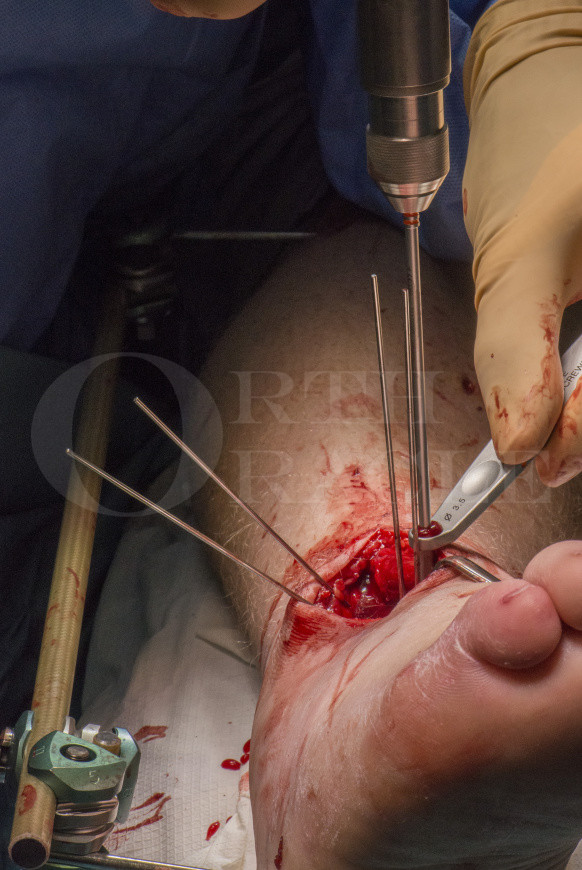
Subscribe to get full access to this operation and the extensive Foot Surgery Atlas.
Learn the Pilon fracture: C-type fixed using Smith and Nephew EVOS small fragment system surgical technique with step by step instructions on OrthOracle. Our e-learning platform contains high resolution images and a certified CME of the Pilon fracture: C-type fixed using Smith and Nephew EVOS small fragment system surgical procedure.
The operative fixation of pilon (pestle) fractures was first described by Ruedi and Allgower in 1968. They advocated surgical fixation with a four stage approach. Firstly restoration of fibula length, secondly reconstruction of the joint surface, thirdly bone grating to the metaphysis and fourthly a medial buttress plate. They also provided a classification with 3 types, Type 1 with no joint displacement, type 2 with articular displacement and type 3 with significant articular comminution.
The majority of the injuries reported by Ruedi and Allogower occurred after skiing accidents, in contrast to this most injuries seen in less mountainous regions occur after high energy injuries involving axial loads to the ankle such as falls from significant height or road traffic accidents. The classification has been further refined by Topliss and Atkins who described the commonly seen articular fragments and patterns of injury.
Current management of high energy pilon fractures normally involves a staged approach with initial placement of an external fixator to resuscitate the soft tissues and restore overall limb alignment followed by definitive fixation once the soft tissues have recovered sufficiently.
Readers will also find of use the following operative techniques on OrthOracle dealing with pilon fractures:
Internal fixation of distal tibial Pilon fracture using Stryker AxSOS 3Ti plate.
Author: Mr Paul Fenton FRCS (Tr & Orth)
Institution: The Queen Elizabeth Hospital, Birmingham, UK.
Clinicians should seek clarification on whether any implant demonstrated is licensed for use in their own country.
In the USA contact: fda.gov
In the UK contact: gov.uk
In the EU contact: ema.europa.eu



















