Polyganics Neurocap application to the geniculate branches of the saphenous nerve
Overview
Subscribe to get full access to this operation and the extensive Upper Limb & Hand Surgery Atlas.
Learn the Polyganics Neurocap application to the geniculate branches of the saphenous nerve surgical technique with step by step instructions on OrthOracle. Our e-learning platform contains high resolution images and a certified CME of the Polyganics Neurocap application to the geniculate branches of the saphenous nerve surgical procedure.
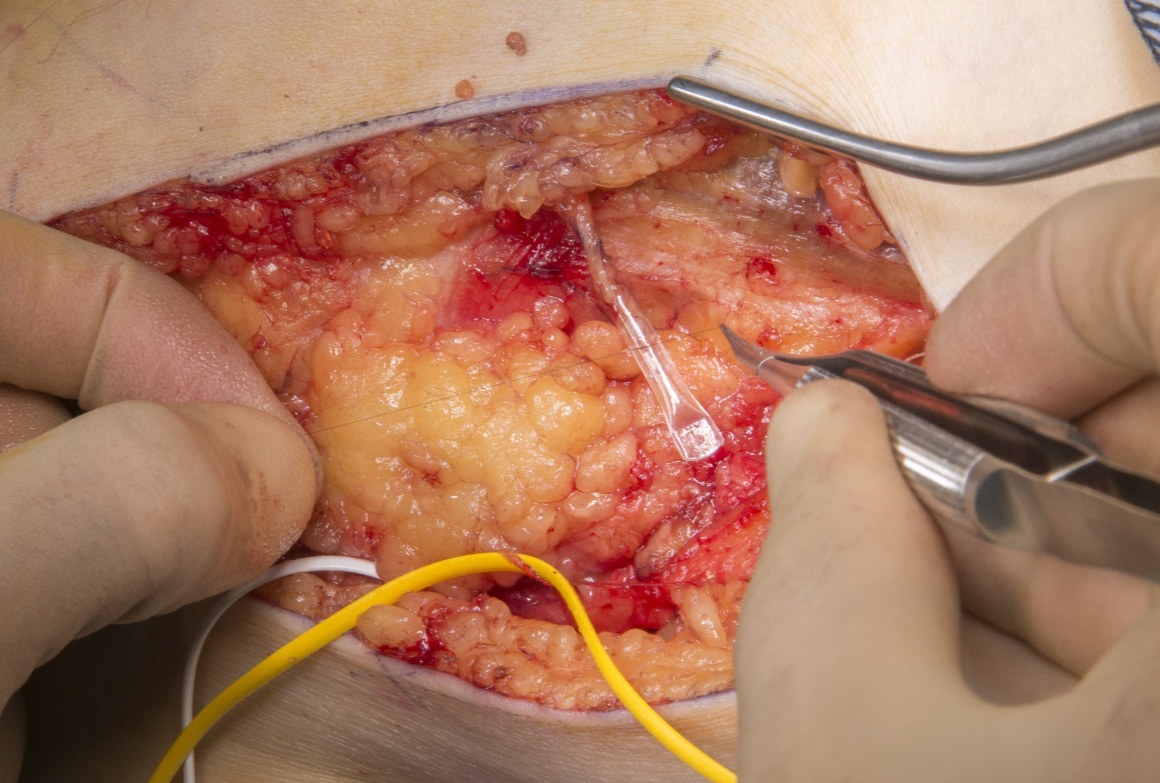
The infra-geniculate branches of the saphenous nerve are susceptible to injury as they cross the anteromedial aspect of the knee and proximal tibia. Injury to these branches may result in anterior knee pain and altered sensation over the proximal and medial tibia. Potential injury should be considered when there is unexplained anterior knee pain. There is usually a positive Tinel’s sign when tapping over the site of the injured nerve. A diagnostic block of the saphenous nerve as it exits Hunter’s canal on the medial thigh is useful in demonstrating that the pain origin is in the saphenous nerve.
Surgical exploration at the point of maximum Tinel’s sign may identify a neuroma. Reconstruction of the injured nerve with autologous nerve graft or processed nerve allograft are options when the distal nerve stump is available. In situations where the distal nerve stump is of poor quality of not available the management of the end neuroma depends on a number of factors including the quality of the surrounding tissues.
Proximal neurectomy of the affected branch is an option, however the nerve stump may have further axon sprouting, resulting in neuroma formation with scar tether that may result in persistent or recurrent symptoms.
The Polyganics BV, Netherlands Neurocap TM is a bioresorbable polycaprolactone capping device that can be placed over the sectioned proximal nerve stump after removal of the end neuroma. The device contains a chamber that allows unsupported axon outgrowth from the proximal stump for a few millimetres in an enclosed environment protected from surrounding scar. The nerve is protected from mechanical and neurotrophic stimulation and eventually atrophies, minimising the risk of a recurrent symptomatic neuroma formation. The cap is broken down, losing mechanical integrity over 3-6 months and finally fully resorbing by 18 months.
The device was evaluated in the STOP Neuroma cohort study and the multi-centre international Protect Neuro study with good reduction in visual analogue pain, reduction in use of analgesia and neuromodulators plus low rates of complication and neuroma recurrence.
Author: Dominic Power FRCS Orth, Consultant Hand and Peripheral Nerve Surgeon
Institution: Peripheral Nerve Injury Service, Queen Elizabeth Hospital, Birmingham, UK
Clinicians should seek clarification on whether any implant demonstrated is licensed for use in their own country.
In the USA contact: fda.gov
In the UK contact: gov.uk
In the EU contact: ema.europa.eu



















