Distal humeral endoprosthesis: Stanmore implants(Stryker) custom implant for revision
Overview
Subscribe to get full access to this operation and the extensive Shoulder & Elbow Surgery Atlas.
Learn the Distal humeral endoprosthesis: Stanmore implants(Stryker) custom implant for revision surgical technique with step by step instructions on OrthOracle. Our e-learning platform contains high resolution images and a certified CME of the Distal humeral endoprosthesis: Stanmore implants(Stryker) custom implant for revision surgical procedure.
Resection of solitary renal osseous metastases has been proven to offer a survival benefit to patients: (https://online.boneandjoint.org.uk/doi/pdf/10.1302/0301-620x.82b1.0820062).
Endoprosthetic replacement following excision of metastases has also been shown to restore and preserve function and relieve pain and is advocated in patients with estimated survival greater than the time to rehabilitate i.e. greater than 6-12 months. Careful patient selection by way of thorough pre-operative clinical and radiological assessment plus liaison with oncology teams is essential to identify those patients most likely to benefit from such major surgery and to exclude those in whom the risks outweighs the potential benefits.
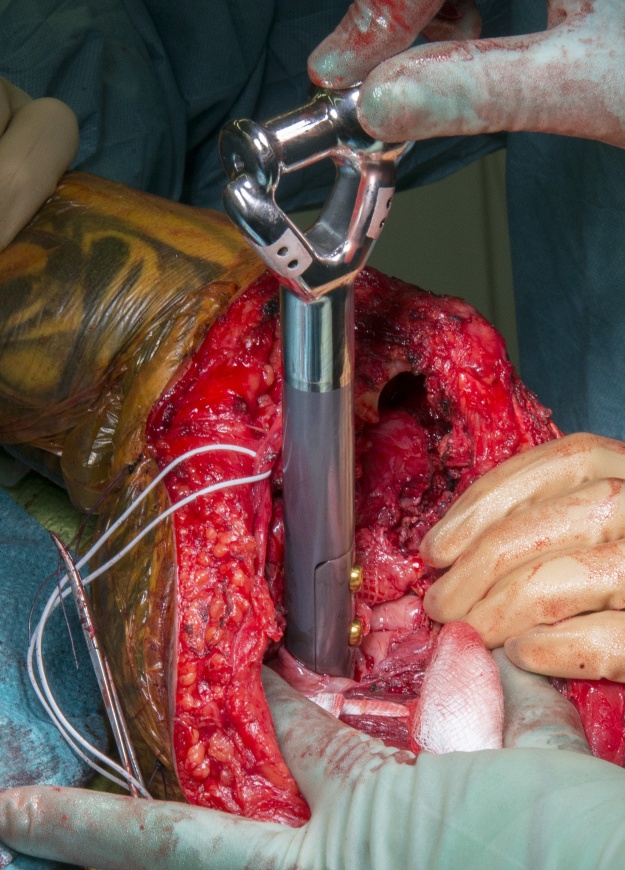
Purely diaphysial bone tumours and intercalary endoprosthetic replacements are rare; at the Royal Orthopaedic Hospital they represent 3% of endoprosthetic replacements. Aseptic loosening is the most common cause of mechanical failure of intercalary endoprosthetic replacements which necessitates challenging revision surgery to preserve function and control pain. Revision of modular intercalary endoprostheses may only require the loose end of the prosthesis to be revised, preserving the integrated portion of the implant, as in this case. (https://www.researchgate.net/profile/Sammy_Hanna2/publication/51213976_Custom_endoprosthetic_reconstruction_for_malignant_bone_disease_in_the_humeral_diaphysis/links/0f31752f6515fdca14000000/Custom-endoprosthetic-reconstruction-for-malignant-bone-disease-in-the-humeral-diaphysis.pdf)
Distal humeral endoprostheses are very uncommon implants. They typically utilise a cemented ulna component and a hinge mechanism to replace the humero-ulna joint, however in this case we were able to preserve the epicondylar attachments for the stabilising ligaments thus only a hemiarthroplasty was required. This implant has a silver coating (Agluna) to prevent prosthetic infection and hydroxyapatite attachment plates for the medial and lateral epicondyles to achieve osseointegration in an attempt to avoid recurrent aseptic loosening.
Author: Mr Jonathan Stevenson FRCS (Tr & Orth)
Institution: The Royal Orthopaedic Hospital, Birmingham, UK.
Clinicians should seek clarification on whether any implant demonstrated is licensed for use in their own country.
In the USA contact: fda.gov
In the UK contact: gov.uk
In the EU contact: ema.europa.eu
Online learning is only available to subscribers.



















