Revision Total Hip replacement: 3D custom dual mobility cup and cemented endoprosthesis with bone impaction grafting
Overview
Subscribe to get full access to this operation and the extensive Hip Surgery Atlas.
Learn the Revision Total Hip replacement: 3D custom dual mobility cup and cemented endoprosthesis with bone impaction grafting surgical technique with step by step instructions on OrthOracle. Our e-learning platform contains high resolution images and a certified CME of the Revision Total Hip replacement: 3D custom dual mobility cup and cemented endoprosthesis with bone impaction grafting surgical procedure.
Revision total hip arthroplasty (THA) in the setting of extensive femoral bone loss poses a considerable challenge to the adult reconstructive surgeon. In the acetabulum, reconstructive options that assist with bone loss include trabecular metal cups and augments, reconstruction rings and custom made implants. When the proximal femoral bone stock is deficient or absent there are also a variety of options for reconstruction, including distal cementless fixation (either modular or non-modular), impaction bone grafting (IBG), megaprosthesis or even allograft prosthesis composite (APC). Each of these procedures has advantages and disadvantages related to bone preservation, the length of the surgery and its complexity.
Dislocation in complex revision THA is a problem which occurs in up to 30% of cases. Techniques to minimise this particular risk include the use of a large femoral head, a constrained acetabular liner, or implanting a dual mobility cup.
Within this presentation, the Paprosky acetabular and the Endoclinik femoral classifications are discussed in detail along with principles of peri-prosthetic joint infection treatment. The surgical techniques covered are spacer removal, acetabular reconstruction using a custom 3D trabecular titanium cup with a dual mobility insert and femoral bone impaction grafting technique using a cemented modular femoral endoprosthesis.
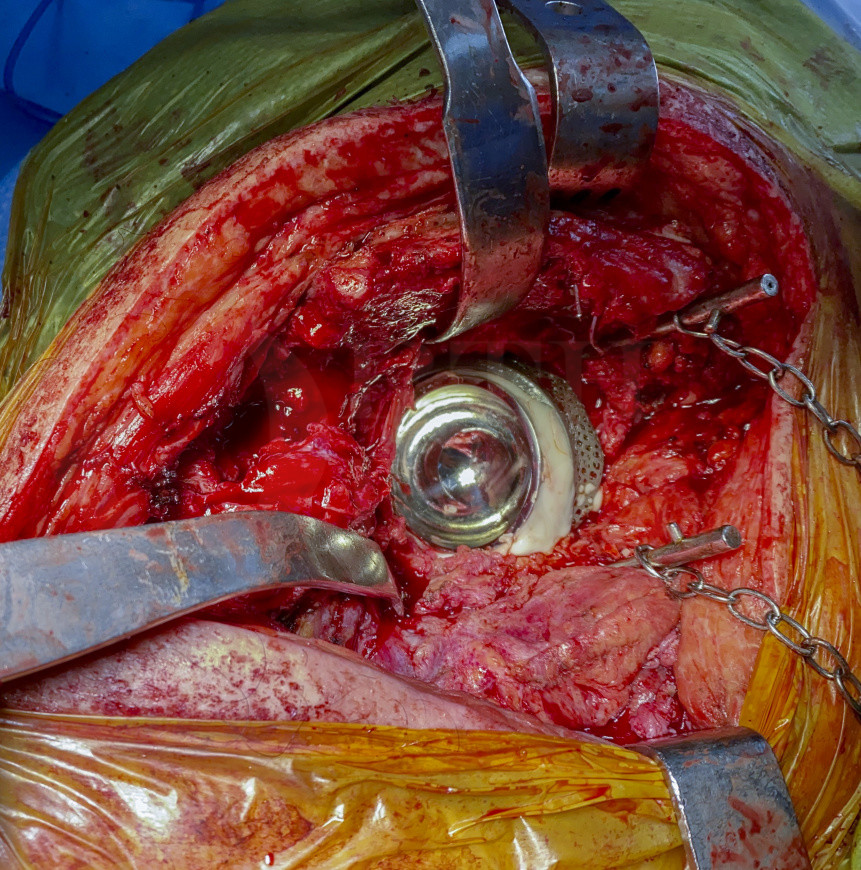
In this particular and complex case, distal uncemented fixation was not possible due to the 30 mm width of the endomedullary canal, an allograft prosthesis composite would have been an unrecommended method due to the potential mismatch between the donor and the host bone. I decided therefore to combine the bone restitution technique of impaction allo-grafting is, using a non-conventional implant in order to achieve primary cemented fixation in a damaged femur lacking cancellous bone, an essential condition to obtain a durable cemented fixation.
A custom made, 3D CT based acetabular model of trabecular titanium was indicated to reconstruct this complex acetabular bone defect (Raomed, Córdoba, Argentina). Once the 3D model has been printed the manufacturing engineers design an initial implant, which is then customized with the surgeon according to the bone quality. The fixation features take account of the bony deficit, bone quality and location of optimum residual bone for fixation.
Due to the massive proximal and endomedullary femoral bone loss, a cemented non-conventional megaprosthesis (OSS, Biomet, USA) was used in combination with the impaction bone grafting technique.
This presentation will allow the Reader to understand the principles and practical steps required for extreme impaction grafting of the femur which is a skill that all revision surgeons should be familiar with.
Readers will find the following associated OrthOracle techniques also of use:
Revision Total Hip replacement: Direct exchange Link MP revision stem for periprosthetic fracture
Author: Martin Buttaro MD
Institution: Hospital Italiano de Buenos Aires, Argentina
Clinicians should seek clarification on whether any implant demonstrated is licensed for use in their own country.
In the USA contact: fda.gov
In the UK contact: gov.uk
In the EU contact: ema.europa.eu