Targeted Muscle Reinnervation (TMR) of Superficial Radial Nerve using Axogen Avance processed nerve allograft
Overview
Subscribe to get full access to this operation and the extensive Upper Limb & Hand Surgery Atlas.
Watch the overview
Learn the Targeted Muscle Reinnervation (TMR) of Superficial Radial Nerve using Axogen Avance processed nerve allograft surgical technique with step by step instructions on OrthOracle. Our e-learning platform contains high resolution images and a certified CME of the Targeted Muscle Reinnervation (TMR) of Superficial Radial Nerve using Axogen Avance processed nerve allograft surgical procedure.
The superficial radial nerve (SRN) is vulnerable to injury in the distal 1/3 of the forearm, wrist and hand. The main nerve trunk emerges from beneath the brachioradialis tendon and the divides into smaller branches that further divide as they pass across the anatomical snuff box to innervate the skin of the dorsum of the thumb, the first webspace and the dorsum of the index and middle fingers.
Injured nerve branches may form neuromas, which are sensitive to local pressure or irritation from contact in this area and even the lightest tough from clothing may result in painful sensations. Avoidance is common and frequently superficial radial neuroma pain is intractable and resistant to neuromodulation therapy or surgical neuroma management.
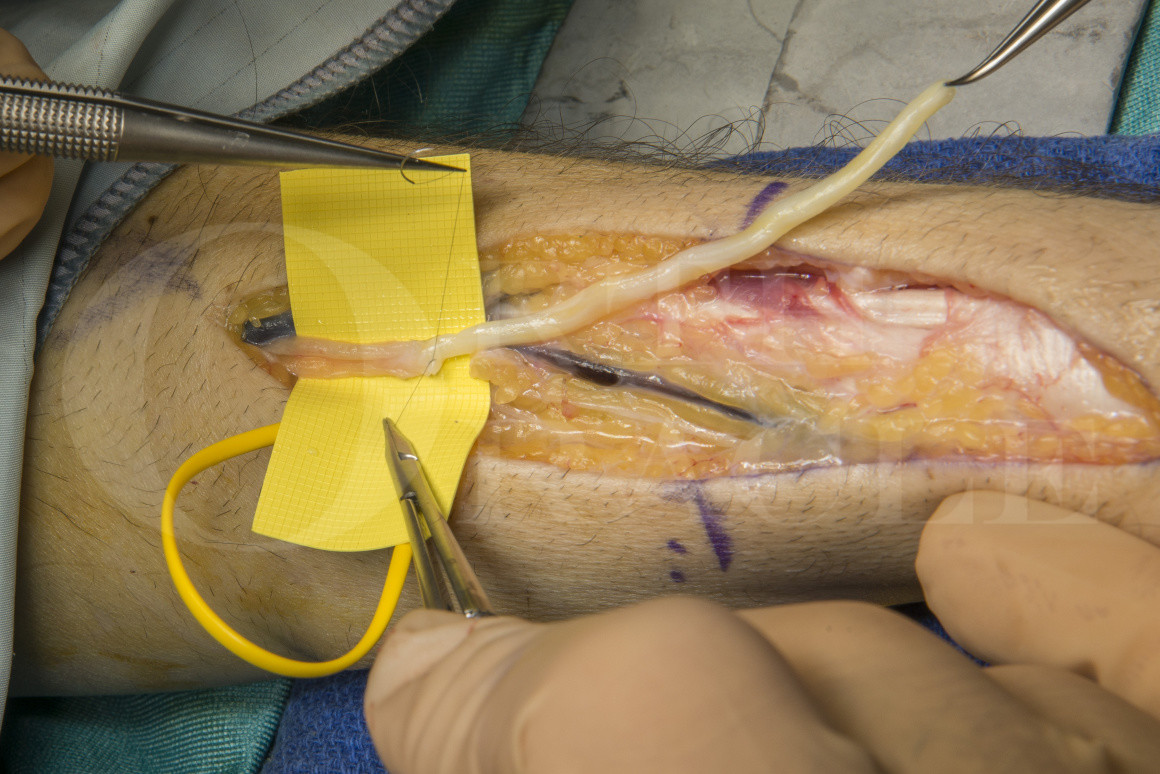
A historic mainstay of surgical management was the neuroma resection and relocation of the proximal stump to an intra-osseous drill hole. The aim was to minimise local irritation and should a recurrent neuroma form, this would be contained within the distal radial bone and therefore protected from further injury. Improved understanding of central neural mapping favours active reconstruction for neuromas, rather than simply resection and burying the stump in an intra-osseous tunnel. This can be achieved through reconstruction of the gap resulting from neuroma resection using either autologous nerve graft, or processed nerve allograft, which is my preference.
The advantage of the allograft is the absence of donor site morbidity in patients with an existing pain driver and pain sensitisation. The technique is only possible though when there is a distal nerve stump to graft to. Successful axonal regeneration may reinnervate the denervated skin, provide physiologic central signalling from the peripheral and enable cortical down regulation of the previous peripheral pain source. Alternatively when a distal nerve stump is not available to reconstruct the divided nerve, the technique of targeted muscle reinnervation (TMR) allows active axonal regrowth along a grafted distal nerve branch to a muscle, and population of the intra-muscular neural plexus with the regenerated axons from the neuroma site to a deep location. During the regenerative phase, the central reprocessing of the afferent signalling reduces the pain perception intensity.
The technique of TMR is proven in prevention of neuromas and phantom pain in primary amputation as well as in secondary intervention for neuroma pain after amputation. In the lower limb, TMR has been used primarily to redirect axons from a mixed motor-sensory nerve stump after transection to a motor branch in the vicinity of the amputation to goo effect. When there are no motor branches available, or the risk of muscle wasting and therefore prosthetic fitting or padding is a concern, then development of a reconstructive peripheral nerve interface (RPNI) may be preferred. Osteointegration may be used to improve prosthetic interface with the skeleton in cases with poor muscle bulk, in short stomps and in cases where there is neuroma pain from a traditional socket interface.
Applying the technique of TMR to the management of superficial radial nerve (SRN) neuromas is an elegant method of achieving active neural regeneration in the absence of a good quality distal nerve stump, either as a primary procedure, or as a salvage after failed SRN neuroma surgery.
The AXOGEN AVANCE processed nerve allograft is human nerve tissue that is processed to remove cellular material and neurotoxic glycoproteins. The nerve tissue is available in a number or lengths (15mm, 30mm, 50mm and 70mm) and diameters (1-2mm, 2-3mm, 3-4mm, 4-5mm). It is screened to reduce the risk of infection transmission and provided sterile and frozen.
There is excellent safety data for use in nerve gap repair as well as efficacy data for digital nerve gap repair, including randomised controlled trial outcomes that are comparable with autologous graft. There also exists good data for non-digital sensory nerve gap repair. The evidence for use in motor and mixed nerve gap repair is more limited and data is collected in the RANGER database which reports on outcomes.
Patients must be informed that it is derived from human nerve tissue.
Readers will also find the following OrthOracle surgical techniques of interest;
Digital nerve reconstruction with Axogen Avance processed nerve allograft
Median nerve neurolysis, resection and reconstruction using Axogen AVANCE processed nerve allograft
Dorsal cutaneous ulnar nerve reconstruction using Axogen Avance nerve allograft
Author: Dominic Power MA MB BChir FRCS Tr & Orth, Consultant Hand and Peripheral Nerve Surgeon
Institution: The Queen Elizabeth Hospital, Birmingham, UK.
Clinicians should seek clarification on whether any implant demonstrated is licensed for use in their own country.
In the USA contact: fda.gov
In the UK contact: gov.uk
In the EU contact: ema.europa.eu



















