Total knee replacement: Vanguard XP cruciate retaining (Zimmer-Biomet)
Overview
Subscribe to get full access to this operation and the extensive Knee Surgery Atlas.
Professional Guidelines Included
Learn the Total knee replacement: Vanguard XP cruciate retaining (Zimmer-Biomet) surgical technique with step by step instructions on OrthOracle. Our e-learning platform contains high resolution images and a certified CME of the Total knee replacement: Vanguard XP cruciate retaining (Zimmer-Biomet) surgical procedure.
Bicruciate retained total knee arthroplasty (TKA) involves the preservation of both the Anterior and Posterior Cruciate Ligaments whilst replacing all three compartments of the knee, patellofemoral and both medial and lateral tibiofemoral.
Early designs of knee replacement(TKR) tried to mimic the native knee and included Bicruciate retaining mono block designs (i.e. one piece tibial baseplate) and separate medial and lateral prostheses in the tibiofemoral joints such as the early Oxford Knee Designs.
In the 1970’s Cloutier designed a bicruciate TKA (the Hermes 2C) which was implanted as an originator series in 163 knees in 130 patients. The results were published at 10 years and 22 years, with 82% implant survival at 22 years and objectively stable knees. There were 12 revisions due to polyethylene failure, but if aseptic loosening was used as the indicator for revision the survival rate was 96% at 22 years. This is better than the current gold-standard for longevity of the fixed bearing cemented PCL retained total knee replacement from a number of designs with 10 year survival around this 95-96% mark.
With the advent of contemporary TKA and partial knee replacement designs bicruciate TKA never reached popularity.
More recently there has been a global recognition that up to 20% of patients following TKA have a problematic pain in their knees. Consequently many avenues are being explored to try and improve outcome. Examples include the development of robotic and navigated knee arthroplasty surgery to multi-disciplinary schemes to manage patients experiencing pain after knee arthroplasty such as the STAR (Support and Treatment After Replacement) research programme in Bristol, UK.
Fo more information on the STAR programme please visit:
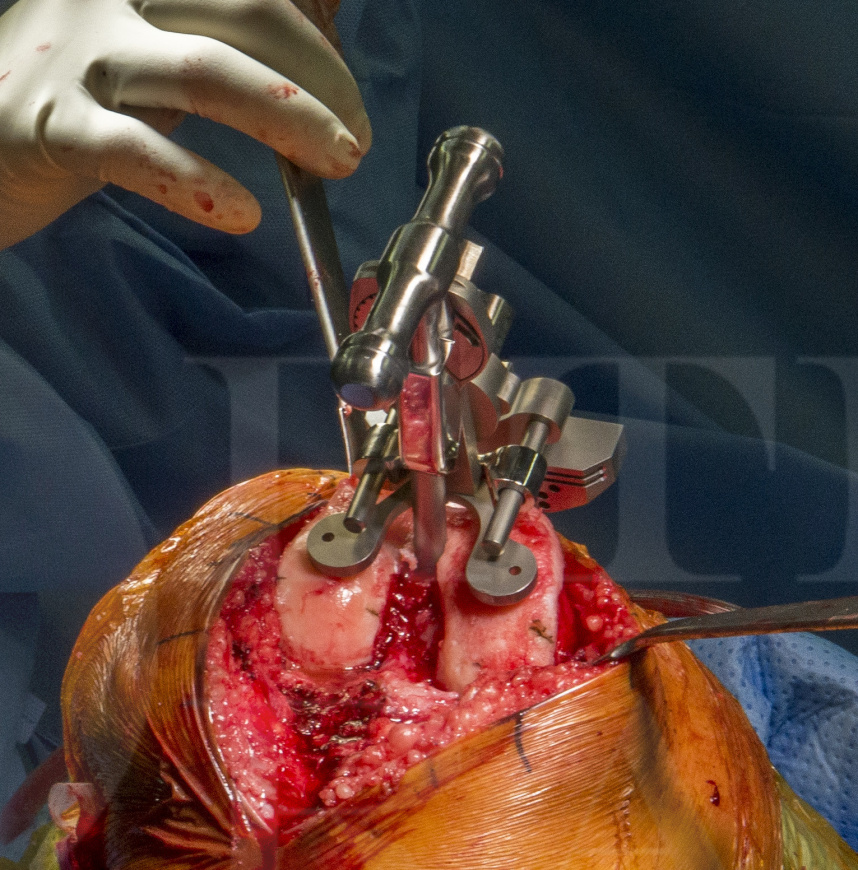
The Vanguard XP is a modified Vanguard PCL retaining TKR which mimics the shape of the Cloutier knee, to avoid resection of the tibial eminence and thus allow retention of the ACL and PCL. The femoral and patella designs are identical to ‘standard’ Vanguard cruciate retained TKA constructs. The tibial component is therefore very different compared to a standard implant because of the ‘cut out’ for the ACL and this also avoids the central keel / stem which is common on most TKA designs. To improve tibial fixation given the lack of stem and keel, the Vanguard XP relies on two small pegs and two small keels on either side of the retained bone island.
One of the risks of bicruciate designed TKAs is fracture of the bone island which then may compromise the ACL. Extreme care must be taken not to under-resect thus over tightening in extension which will fracture the ACL island.
Given the novel design and limited outcome studies about bicruciate knee replacement, all of the cases I have performed are included on the Beyond Compliance web site (https://www.beyondcompliance.org.uk/). This platform, which pools surgeons cases, allows careful monitoring of any cohort and optimises the chance of early warning signs being detected if issues become evident with an implant. In addition many of these cases were part of the ALLIKAT trial which is an RCT of Bicruciate retained versus PCL retained TKA, comparing the Vanguard XP with the CR implant; the details of this are available through clinicaltrials.gov and the Health Research Authority (SRCTN12584521). In summary there is a 1:1 RCT and a separate cohort of Vanguard XP patients.
For more information on Beyond Compliance visit:
https://www.beyondcompliance.org.uk/product.aspx?pid=7357
For more information on the ALLIKAT trial visit:
https://clinicaltrials.gov/ct2/show/NCT03302013
OrthOracle readers will also find of interest the following associated instructional techniques:
Total Knee replacement: Zimmer Biomet Nexgen rotating hinge replacement
Total Knee replacement: Mako Triathlon robotic assisted cruciate retaining TKR (STRYKER)
Total Knee replacement: MAKO robotic triathlon cruciate substituting knee replacement
Total knee replacement Genesis 2 (PS) with bi-convex patella (Smith and Nephew)
Total knee replacement-Triathlon (Stryker) posterior stabilised knee.
Total knee replacement (posterior stabilised): Visionaire Genesis II (Smith and Nephew)
Total Knee Replacement: De Puy Attune implant
Total Knee Replacement: Vanguard cruciate retaining knee replacement(Zimmer-Biomet)
Total Knee replacement: Vanguard 360 knee replacement (Zimmer-Biomet)
Author: James Murray
Institution: Avon Orthopaedic Centre, Southmead Hospital, Bristol, UK
Clinicians should seek clarification on whether any implant demonstrated is licensed for use in their own country.
In the USA contact: fda.gov
In the UK contact: gov.uk
In the EU contact: ema.europa.eu
Online learning is only available to subscribers.



















