Achilles tendon lengthening: open
Overview
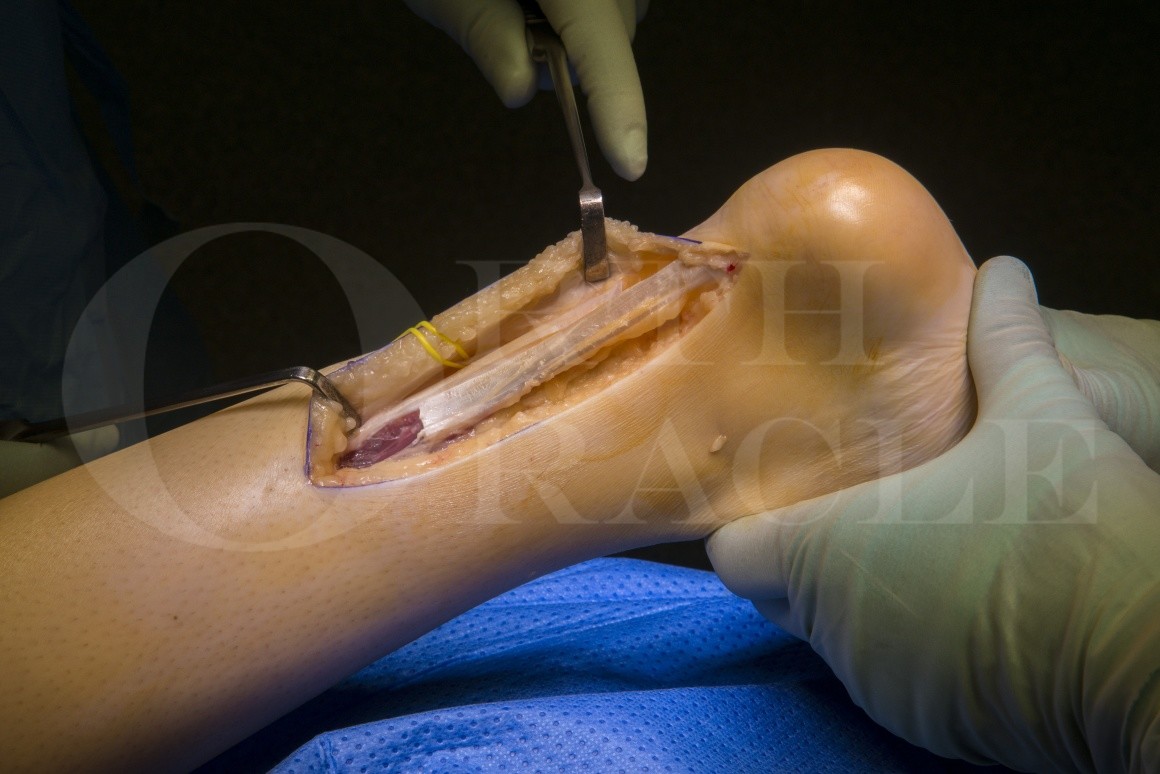
Subscribe to get full access to this operation and the extensive Foot Surgery Atlas.
Learn the Achilles tendon lengthening: open surgical technique with step by step instructions on OrthOracle. Our e-learning platform contains high resolution images and a certified CME of the Achilles tendon lengthening: open surgical procedure.
An acquired equinus contracture of the ankle has a number of more common causes. Despite early bracing and functional rehabilitation it can occur after traumatic or ischemic brain injury, spinal cord injury, ischaemic muscle contracture or as part of a more generalised neurological condition.
Recovery specifically following traumatic or ischaemic brain and spinal cord injury is variable. In those whose function is sufficient enough to allow standing or walking, the equinus contracture can become a limiting factor in their rehabilitation. Whilst equinus deformities can be caused by a variety of muscle contractures and joint contractures, normally the achilles-gastrocnemius-soleus complex is the first to develop the fixed deformity. Thus in those cases that present within 2-3 years of a contracture developing an isolated achilles release is usually sufficient to correct the deformity.
The restriction caused by a contracted Achilles tendon will usually become apparent within 2 years of the neurological insult, and as such, this patient population more often have not yet developed significant contracture of their long flexors and joint capsules, when compared to the more chronic neurological or pediatric equinus contractures, or the ischemic muscle contracture group.
Patients with ischemic or traumatic brain injury should be managed in a multi-disciplinary environment, and will require input from Neurologists and Neuro-rehabilitation therapists, Occupational therapists and of course the most important part of any team, the Orthopaedic surgeons.
Author: Mr Nick Cullen, FRCS (Tr & Orth).
Institution: The Royal National Orthopaedic hospital, Stanmore, UK.
Clinicians should seek clarification on whether any implant demonstrated is licensed for use in their own country.
In the USA contact: fda.gov
In the UK contact: gov.uk
In the EU contact: ema.europa.eu



















