Closed reduction and K wiring of 5th carpometacarpal fracture-dislocation
Overview
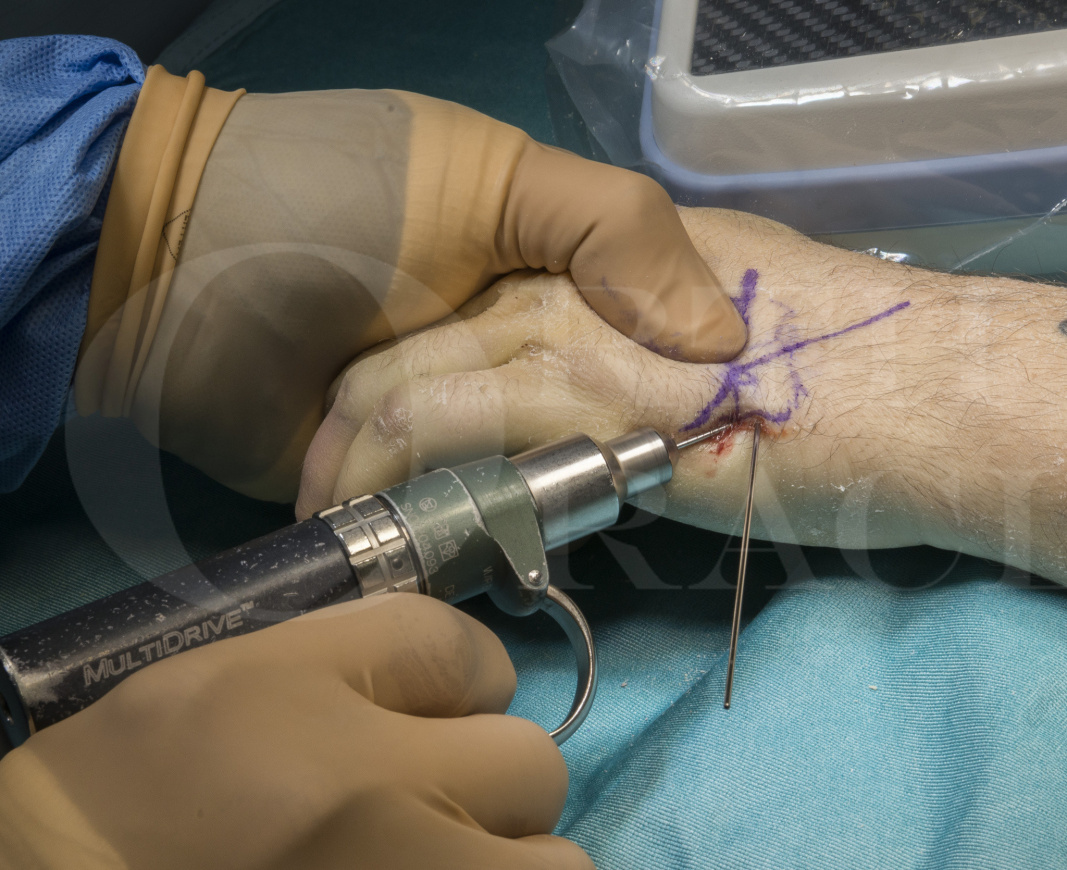
Subscribe to get full access to this operation and the extensive Upper Limb & Hand Surgery Atlas.
Learn the Closed reduction and K wiring of 5th carpometacarpal fracture-dislocation surgical technique with step by step instructions on OrthOracle. Our e-learning platform contains high resolution images and a certified CME of the Closed reduction and K wiring of 5th carpometacarpal fracture-dislocation surgical procedure.
A fracture dislocation of the 5th carpo-metacarpal joint is the second most common injury in the hand, after the thumb carpo-meatcarpal joint. The injury is caused by an axial loading force, most commonly seen in punching injuries. Given this they are common in adult males of a younger age group, may present with associated dirty puncture wounds to the metacarpo-phalangeal joints and may present with or without associated fracture. Most often they are closed injuries and require reduction and stabilisation.
An axial loading force to a clenched fist causes these injuries. The loading vector often results in a metacarpal neck fracture. The force can alternatively be transmitted proximally at the CMC joint resulting in its fracture-dislocation. The bases of the 4th and 5th metacarpals articulate with the hamate and are stabilized by dorsal and palmar intermetacarpal ligaments along with the interosseous ligament. The deforming force causes the metacarpal base to subluxate or dislocate with or without an avulsion fragment from the hamate. Occasionally, there may be an associated fracture involving the base of the metacarpus. The radial 25% of the metacarpal base may remain intact in its normal anatomical location. In this respect, this injury is not dissimilar to a Bennet’s fracture at the base of the thumb. Once displaced, the deformity is accentuated by the pull of the extensor and flexor carpi ulnaris as well as the abductor digiti minimi.
Author: Mr Manish Gupta FRCS (Tr & Orth), Consultant hand surgeon
Institution: The Queen Elizabeth hospital, Birmingham, UK.
Clinicians should seek clarification on whether any implant demonstrated is licensed for use in their own country.
In the USA contact: fda.gov
In the UK contact: gov.uk
In the EU contact: ema.europa.eu



















