Medial Femoral Condyle Focal Resurfacing with HemiCAP(Arthrosurface)
Overview
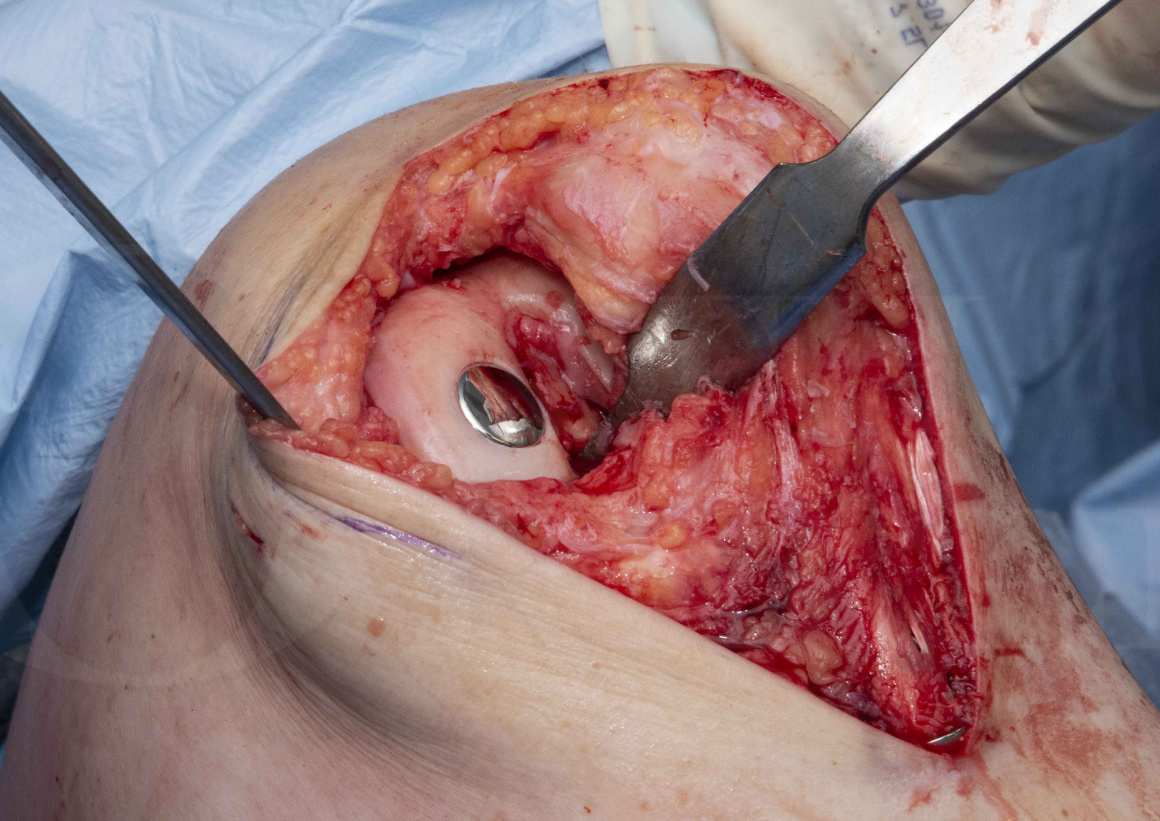
Subscribe to get full access to this operation and the extensive Knee Surgery Atlas.
Learn the Medial Femoral Condyle Focal Resurfacing with HemiCAP(Arthrosurface) surgical technique with step by step instructions on OrthOracle. Our e-learning platform contains high resolution images and a certified CME of the Medial Femoral Condyle Focal Resurfacing with HemiCAP(Arthrosurface) surgical procedure.
Focal Resurfacing is gaining popularity for the treatment of focal articular defects up to 20x20mm in the knee (when using HemiCAP), providing a rapid recovery and no post-operative restrictions, differentiating this from biological reconstructive techniques such as cartilage grafting or the use of scaffolds such as Chondrogide . Biological reconstruction techniques usually require a period of reduced weightbearing up to 2 months and often a restricted range of movement range using a brace. Similarly the lack of post-operative restriction to activity differentiates focal resurfacing from traditional arthroplasty or even partial knee arthroplasty for the usually middle-aged patient with focal chondral damage.
The classic patient for focal resurfacing is in the 40-60 age range with a focal chondral lesion which is symptomatic and has failed traditional non-operative treatments such as offloader bracing and injection therapies such as visco-supplementation or steroid. Please remember to check alignment as osteotomy is an excellent proven treatment for the mal-aligned overloaded joint; focal resurfacing can be considered with or after osteotomy.
The HemiCAP was released in 2003, but it has taken some time to ‘catch on’ probably due to its innovative approach using a metal articular ‘cap’ inset within the articular surface. This is fundamentally different from partial knee replacement where there is a partial replacement on both sides of a joint.
There are now a number of implants available such as the HemiCAP, however it has the longest clinical experience and evidence base. Alternatives are the Bio-Poly, a cemented focal resurfacing which uses a novel combination of hyaluronic acid and polyethylene or the Epi-Sealer, a custom-made focal resurfacing relying on uncemented fixation with an option to cover defects up to 25mm in diameter.
All the focal resurfacings are designed to encourage the native chondral surrounding tissue to overgrow the margins of the inset implant.
The ArthroSurface HemiCAP condylar implant provides a surgical treatment for the painful chondral or osteochondral defect in the femoral condyles and has been in production since 2003. However the literature is sparse and as yet there is no independent guidance on its indications from the UK National Institute for Clinical Excellence (NICE) although this is planned a the time of writing (May 2021).
Since the initial design and release in 2003, there have been many evolutions of the design to allow for treatment of the patello-femoral joint (the ‘Wave’ or ‘Kahuna’ implant), the shoulder and the great toe.
The HemiCAP is an uncemented design and relies on cancellous fixation of the screw in the femoral condyle (the ‘fixation device’) and then the impaction of an Articular Component which matches the native anatomy via two sizes (15 and 20mm diameter) over 16 different curvatures. The two components combine with a morse taper.The Articular Component is made of Cobalt Chromium Alloy (Co-Cr-Mo) whilst the undersurface is Titanium plasma spray-coated and the morse taper is Titanium alloy (Ti-6Al-4V).
The largest metanalysis gives a revision rate of 9% at 4 years, but this includes all focal resurfacings and there have been longer terms studies at 7 years mean showing better outcome with condylar focal resurfacing. There is no doubt that at present the literature is still sparse, but all of the papers have been positive suggesting functional improvement in the vast majority of cases with a small reoperation and even lower revision rate.
At present focal resurfacings are not included in the National Joint Registry in the UK, but this is currently being debated and the relevant national bodies have been asked to comment on their future inclusion in the NJR. Please see the results section for the list of papers and summary results.
Author: James Murray FRCS (TR & Orth)
Institution: The Avon Orthopaedic centre, Southmead Hospital, Bristol UK
Clinicians should seek clarification on whether any implant demonstrated is licensed for use in their own country.
In the USA contact: fda.gov
In the UK contact: gov.uk
In the EU contact: ema.europa.eu



















