Mortons Neuroma Excision
Overview
Subscribe to get full access to this operation and the extensive Foot Surgery Atlas.
Learn the Mortons Neuroma Excision surgical technique with step by step instructions on OrthOracle. Our e-learning platform contains high resolution images and a certified CME of the Mortons Neuroma Excision surgical procedure.
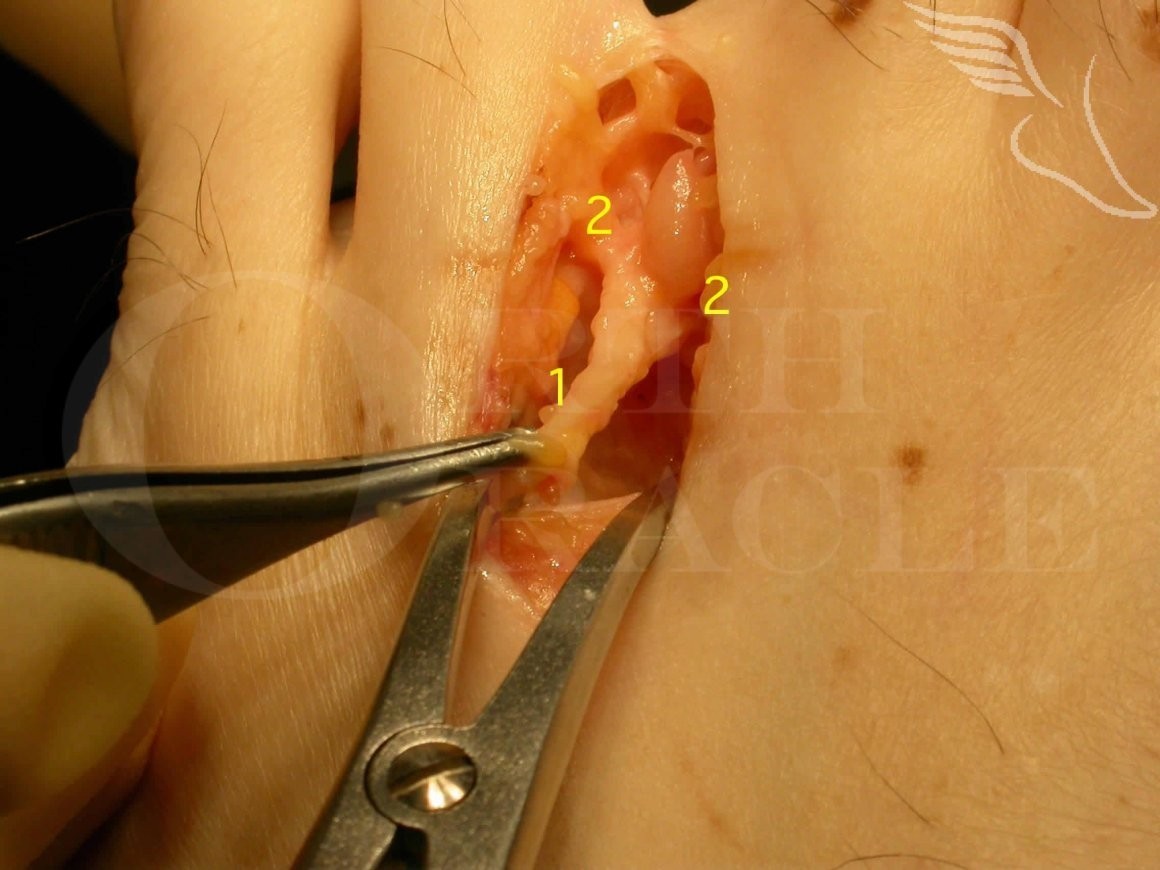
There are a limited number of pathologies which present with pain in the 2/3 or 3/4 inter-metatarsal area, a mortons’ neuroma being one of the commoner ones. Others are mechanical pain from the metatarsal head, MTP joint synovitis or least commonly degenerative change of the MTP joint.
Pain may be located purely under the effected metatarsal heads or may radiate into the toes and be associated with numbness in the web space or toes. On occasion splaying will effect the position of the toes in the effected web-space. There is a well recognised association between Mortons neuroma and plantar plate tears, leading to cross-over toe deformities. One method of surgically treating this is detailed on OrthOracle at https://www.orthoracle.com/library/crossover-second-toe/
The first line of management of a Mortons’ neuroma is usually non-constricting and supportive shoe-wear with a low threshold for steroid and local infiltration to reverse the reactive swelling of the digital nerve that comprises a neuroma. The injection may be performed with or without ultrasound guidance, though intercurrent pathologies such as MTP joint synovitis or plantar plate tears are not confirmed without imaging being used. MRI can also be used to diagnose the condition.
Surgical excision is highly effective, though not invariably successful, for treatment of the intrusive pain associated with the condition but minor degrees of residual associated symptoms are not uncommon post-operatively and realistically the forefoot takes 6 to 8 weeks to resolve after such surgery.
Author: Mark Herron, FRCS.
Institution: The Wellington Hospital, London, UK.
Clinicians should seek clarification on whether any implant demonstrated is licensed for use in their own country.
In the USA contact: fda.gov
In the UK contact: gov.uk
In the EU contact: ema.europa.eu



















