Proximal Row Carpectomy (for advanced arthritic wrist pain)
Overview
Subscribe to get full access to this operation and the extensive Upper Limb & Hand Surgery Atlas.
Learn the Proximal Row Carpectomy (for advanced arthritic wrist pain) surgical technique with step by step instructions on OrthOracle. Our e-learning platform contains high resolution images and a certified CME of the Proximal Row Carpectomy (for advanced arthritic wrist pain) surgical procedure.
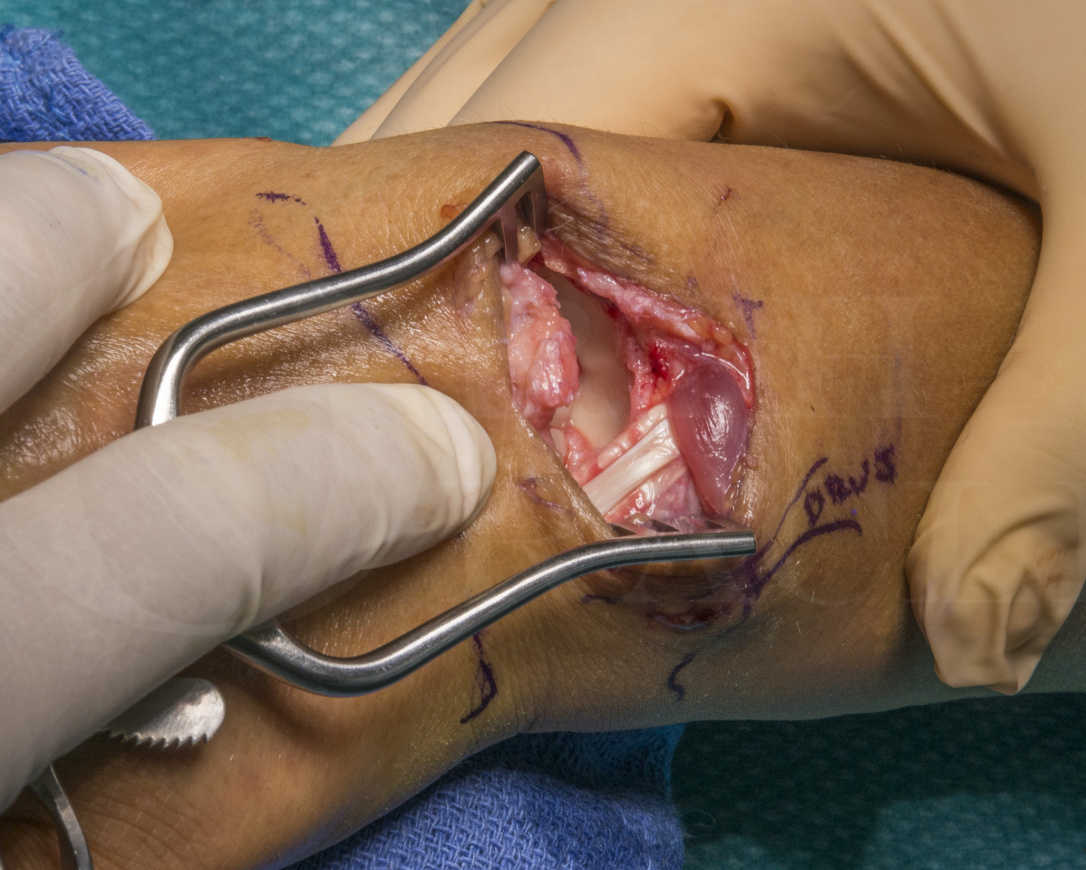
This is a detailed step by step instruction through a proximal Row Carpectomy (PRC), a procedure in which the proximal row of carpal bones (Scpahoid, Lunate and Triquetrum) are removed.
The PRC is a salvage operation usually undertaken for wrist pain when the scaphoid fossa is damaged and arthritic. The most common cause for this is a Scapho-Lunate advanced collapse (SLAC) stage 2. The procedure can also be used for Keinbock’s disease, proximal carpal row instability resistant to initial surgery or if the wrist needs to be shortened due to trauma or Volkmann’s ischemic contracture.
The critical point in planning this surgery is that the lunate fosse of the radius and the proximal capitate must be free from damage.
During the surgery the wrist capsule is tightened and allowed to scar up to create a stable but mobile Neo-wrist joint.
Following a period of 6-8 weeks in plaster cast and then rehabilitation, it is expected the patient will achieve around 50% of their normal range of movement.
Once the wrist is fully healed and strengthened around 6 months post surgery then heavy work is possible although the range of motion and grip strength will be limited to around 50% and 65% respectively.
One of the common alternative treatments to PRC is a four corner fusion. The advantage of a PRC over the technique is a slightly increased post operative range of movement, a lack of a need for metalwork, the PRC is less technically demanding and no bone healing required, an advantage in the unrepentant smoker.
Readers will also find of interest the OrthOracle instructional technique Four Corner carpal Fusion using Medartis plate and scaphoid excision
Author: Mark Brewster FRCS (Tr & Orth)
Institution: The Royal Orthopaedic hospital, Birmingham, UK.
Clinicians should seek clarification on whether any implant demonstrated is licensed for use in their own country.
In the USA contact: fda.gov
In the UK contact: gov.uk
In the EU contact: ema.europa.eu
Online learning is only available to subscribers.



















