Total knee replacement Genesis 2 (PS) with bi-convex patella (Smith and Nephew)
Overview
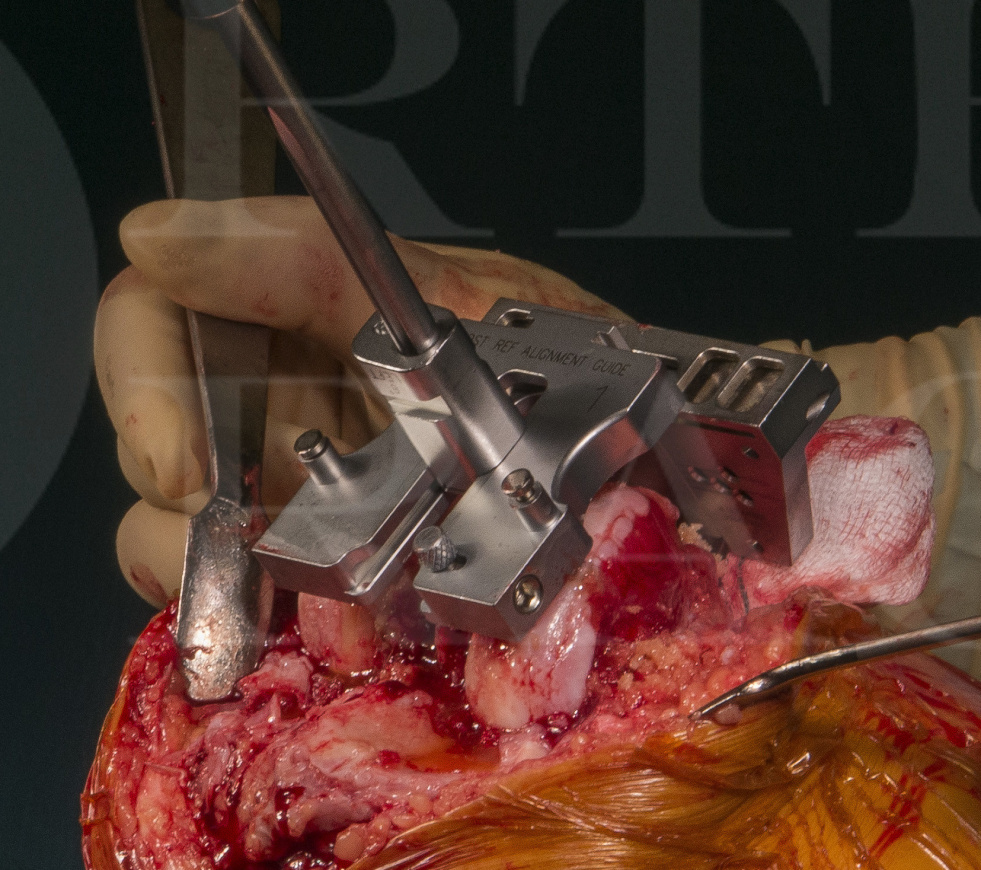
Subscribe to get full access to this operation and the extensive Knee Surgery Atlas.
Learn the Total knee replacement Genesis 2 (PS) with bi-convex patella (Smith and Nephew) surgical technique with step by step instructions on OrthOracle. Our e-learning platform contains high resolution images and a certified CME of the Total knee replacement Genesis 2 (PS) with bi-convex patella (Smith and Nephew) surgical procedure.
The Genesis II knee replacement is a popular bicondylar TKR design which has a proven track record. Dating from the mid 1990s, the Gen II was designed by James Rand, Bob Bourne and Richard Laskin, to incorporate a number of specific design features to optimise performance.
The key benefits of the Genesis II are:
- Asymmetric posterior femoral condyles – the medial posterior condyle was reduced from 9.5mm to 7mm thick to create an ‘external rotation’ of the femoral component relative to the tibia, without changing the trochlea position by physically rotating the component externally relative to the femoral bone. This improves femorotibial contact geometry throughout range as well as optimising patella tracking.
- The coronal geometry is rounded at its edges to improve femoro-tibial contact further and also reducing edge loading which subsequently reduces wear.
- The trochlea groove is sigmoid shaped to allow the patella to be ‘met’ by the femoral component laterally and as the patella medialises on its journey to the tibial tubercle, it is supported underneath by the trochlea of the femoral component.
- The tibial baseplate is asymmetric to optimise tibial coverage for improved sizing and fixation, thus allowing transfer of load through the whole tibial plateau.
- The tibial stem is offset medially from the centre of the tibial component to mirror the native anatomic metaphyseal – diaphyseal mismatch of most patients, which on average is 3mm.
- The Genesis II system was introduced with Cruciate retaining, quickly adding posterior stabilised and there are both uncemented and cemented options
- The Genesis II is available with Cobalt Chrome or Oxinium Femoral components; the Oxinium bearing surface has been shown to reduce where rates in vitro and be clinically safe at 10 years in patients.
- Polyethylene tibial liners are available in standard or highly crosslinked forms (Ultra High Molecular Weight Polyethylene), although the high flexion liner is only available in UHMWPE.
In terms of which variant of Genesis II to choose, I have settled on cemented fixed bearing posterior stabilised with cobalt chrome femoral components for ‘standard’ TKRs. I will always try and perform a partial knee replacement if possible, but once I have made the decision that the whole joint is involved I chose to resurface the patella in all patients. For this I choose the biconvex inset Genesis patella as this allows careful fine tuning of patella construct thickness as described in this operation technique and also the Orthoracle technique Smith and Nephew Journey patello-femoral replacement .
The Biconvex Inset Patella has a 2.3% revision rate at 10 years according to Erak et al; they found that in a series of 521 inset patellae, in 431 patients, there were 14 revisions at 10 years which equates to a 2.7% revision rate. However in the same study at 10 years post implantation, in non-revised patients, there was only a 7.8% incidence of anterior knee pain which is a very low level.
In younger higher demand patients I use the Oxinium femoral component with UMWPE High Flex polyethylene liners; the age bracket for change is around 60-65 years of age in my opinion but this is more dependant on biological age, weight and function of the patient.
OrthOracle readers will also find of interest the following associated instructional techniques:
Total Knee replacement: Zimmer Biomet Nexgen rotating hinge replacement
Total Knee replacement: Mako Triathlon robotic assisted cruciate retaining TKR (STRYKER)
Total Knee replacement: MAKO robotic triathlon cruciate substituting knee replacement
Total knee replacement: Vanguard XP cruciate retaining (Zimmer-Biomet)
Total knee replacement-Triathlon (Stryker) posterior stabilised knee.
Total knee replacement (posterior stabilised): Visionaire Genesis II (Smith and Nephew)
Total Knee Replacement: De Puy Attune implant
Total Knee Replacement: Vanguard cruciate retaining knee replacement(Zimmer-Biomet)
Total Knee replacement: Vanguard 360 knee replacement (Zimmer-Biomet)
Author: James Murray FRCS
Institution: The Avon Orthopaedic Centre, Southmead Hospital, Bristol, UK
Clinicians should seek clarification on whether any implant demonstrated is licensed for use in their own country.
In the USA contact: fda.gov
In the UK contact: gov.uk
In the EU contact: ema.europa.eu



















