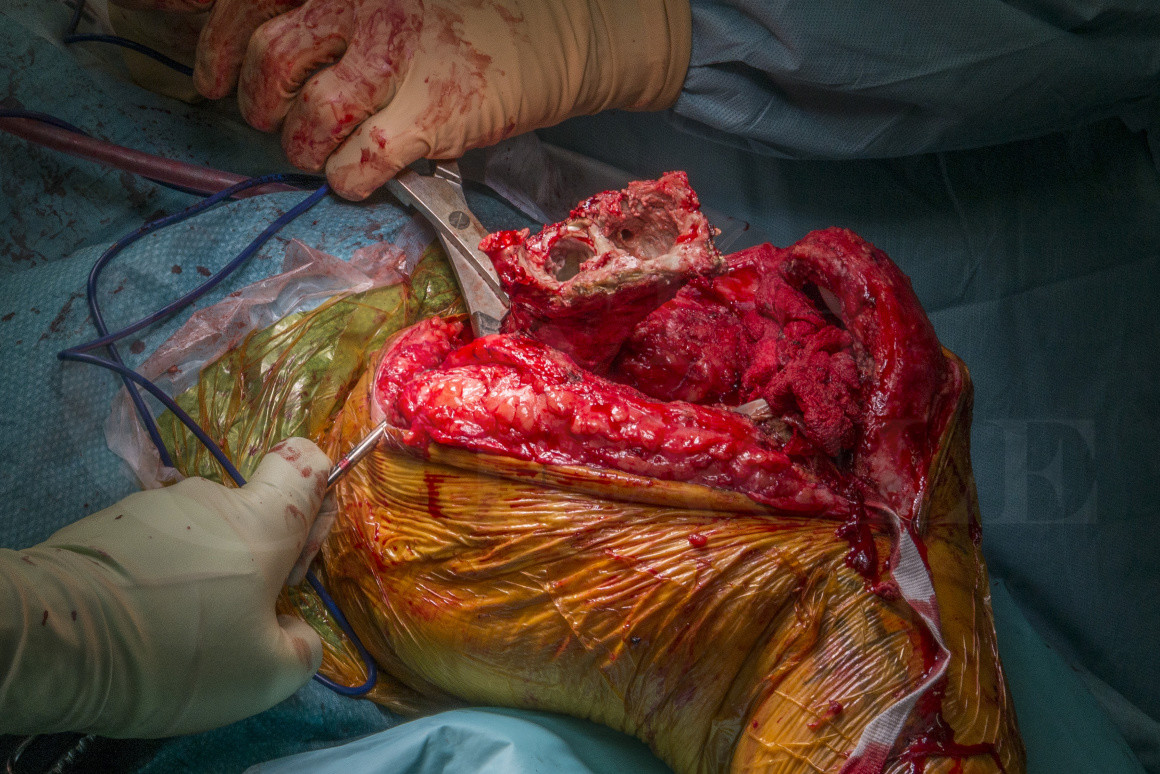
First stage re-revision total knee replacement of Implantcast MUTARS MK EPR using reinforced static antibiotic cement spacer
Overview
Subscribe to get full access to this operation and the extensive Knee Surgery Atlas.
Professional Guidelines Included
Learn the First stage re-revision total knee replacement of Implantcast MUTARS MK EPR using reinforced static antibiotic cement spacer surgical technique with step by step instructions on OrthOracle. Our e-learning platform contains high resolution images and a certified CME of the First stage re-revision total knee replacement of Implantcast MUTARS MK EPR using reinforced static antibiotic cement spacer surgical procedure.
Increasing numbers of primary and revision knee replacements inevitably lead to more prosthetic joint infections (PJI) presenting to specialist PJI multi-disciplinary teams. Infection is a devastating complication of total joint arthroplasty and the most common cause for early failure of joint replacements and most common cause for failure of revision knee replacements at any time. That PJI is associated with higher mortality (than some common malignancies) has been widely reported; five year survival after PJI is 78% compared to 90% in patients undergoing aseptic revision arthroplasty (Matar H, et al. Septic Revision Total Knee Arthroplasty Is Associated With Significantly Higher Mortality Than Aseptic Revisions: Long-Term Single-Center Study (1254 Patients). Journal of Arthroplasty 2021 https://doi.org/10.1016/j.arth.2021.01.068).
The treatment of prosthetic joint infection typically requires surgery involving explant of the infected prosthesis, radical debridement and then either immediate reimplantation or use of an antibiotic loaded cement spacer and delayed reimplantation i.e. a two-stage revision, as in this case. Alternative strategies include debridement and implant retention with modular exchange (indicated in acute PJI) and single-stage revision (considered in infected primary arthroplasty implants, sensitive organisms and without soft-tissue defects requiring plastic surgery). There is endless debate about selecting the correct option for each case, the decision is multi-factorial and probably best decided in specialist PJI MDTs.
Infected revision knee replacements are increasing in prevalence due to the increasing numbers of revision joint replacements being performed for septic and aseptic indications such that a 7.5-fold increase in re-revision knee replacements due to infection that has been experienced in the UK since 2005 (Lenguerrand et al. Description of the rates, trends and surgical burden associated with revision for prosthetic joint infection following primary and revision knee replacements in England and Wales: an analysis of the National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. BMJ Open 2017;7:e014056. doi: 10.1136/bmjopen-2016-014056). Dependent upon the type of host status, repeat two-stage revision for re-infected knee replacements has proven to be possible, although in immunocompromised hosts with poor soft-tissues, amputation should be considered. These limb-salvage procedures are challenging for numerous reasons including segmental bone loss, poor residual bone stock, poor soft-tissues and (usually) medically compromised patients. The outcomes of repeat two-stage revision are not widely described; our hospital data estimates that failure due to recurrent infection after 2 years is 50%, compared to 10% in primary two-stage revisions of infected primary knee replacements, although with repeated surgery the limb-salvage rate exceeds 95%. Failure to control PJI leads to further surgery, antibiotic suppression or amputation.
Here I present a challenging case of PJI in a previously revised TKR with expected segmental femoral and meta-epiphyseal tibial bone loss necessitating an extended tibial osteotomy to explant the infected prosthesis and resection of the distal femur. I demonstrate a technique using overlapping Kuntscher nails (slotted intramedullary nails, first used in WWII) to reinforce static antibiotic loaded cement spacers to stabilise the knee and deliver local antibiotics to the joint cavity, which is a cost-effective method for temporary static spacers with segmental bone loss.
OrthOracle readers will also find the following techniques of interest:
Revision total Knee Replacement- Legion Rotating Hinge Knee ( Smith and Nephew)
Revision total Knee Replacement: Legion CCK (Smith and Nephew)
Author: Jonathan Stevenson FRCS (Tr & Orth)
Institution: Royal Orthopaedic Hospital, Birmingham, UK
Clinicians should seek clarification on whether any implant demonstrated is licensed for use in their own country.
In the USA contact: fda.gov
In the UK contact: gov.uk
In the EU contact: ema.europa.eu