Stem cell harvest and transplant for knee osteochondral defect (Synergy Medical technologies)
Overview
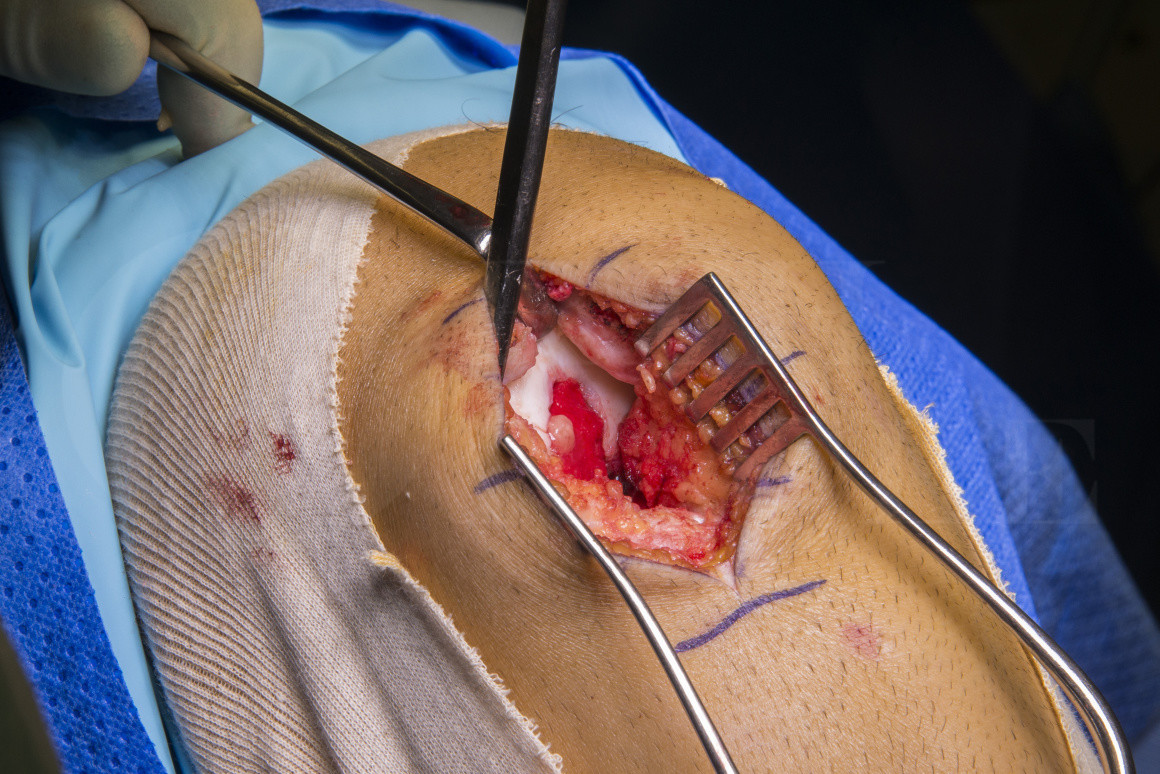
Subscribe to get full access to this operation and the extensive Knee Surgery Atlas.
Professional Guidelines Included
Learn the Stem cell harvest and transplant for knee osteochondral defect (Synergy Medical technologies) surgical technique with step by step instructions on OrthOracle. Our e-learning platform contains high resolution images and a certified CME of the Stem cell harvest and transplant for knee osteochondral defect (Synergy Medical technologies) surgical procedure.
Osteochondral defects (OCDs) of the knee are a relatively common problem and can be challenging to treat. Articular cartilage is avascular and consequently has limited regenerative potential. The avascularity is speculated to limit progenitor cell infiltration, which is necessary for cartilage regeneration. Adequate treatment of OCDs is essential to prevent progressive tissue loss and ultimately degenerative joint disease.
Traditional methods of treatment such as microfracture or mosaicplasty are generally reserved for smaller defects (i.e. < 2 cm2) and result in fibrocartilage formation, which has inferior biomechanical and biochemical properties to hyaline cartilage and over time undergoes degeneration.
The use of a cellular repair (autologous chondrocyte implantation) is not new but recently became a NICE (National Institute for Clinical Excellence) recommended procedure in the UK. It involves two stages and laboratory culture of harvested chondrocyte cells.
This ‘stem cell harvest and transplant’ technique uses chondrocyte precursor cells (mesenchymal stem cells) harvested from the pelvis and has the advantage of being performed in a single stage; it is also cheaper than ACI. We have performed this at our institution for over 4 years and have demonstrated significant improvements in Cincinatti scores at two years. The three year graft survival is 96.3%. Poorer results are found in older patients and those who have had previous surgery.
Rehabilitation is crucial and return to sports is not expected before the six month mark.
The ‘Syngenit surgical’ kit is produced by Synergy Medical Technologies, UK. It comes sterile packed in one box and is based on a similar technique published by Buda et al at the Rizzolli Institute (Buda et al. J Bone Joint Surg Am 2010;92 [Suppl. 2]:2–11). They successfully used a hyaluronic acid membrance and 2mls of concentrated bone marrow aspirate for knee articular cartilage repair.
Readers will also find of interest the following related OrthOracle techniques:
Knee arthroscopy and microfracture of osteochondral defect
Osteochondral grafting of the talus (OATS procedure)
Clinicians should seek clarification on whether any implant demonstrated is licensed for use in their own country.
In the USA contact: https://www.fda.gov/medical-devices/products-and-medical-procedures
In the UK contact: https://www.gov.uk/government/organisations/medicines-and-healthcare-products-regulatory-agency
In the EU contact: https://www.ema.europa.eu/en/human-regulatory/overview/medical-devices
Author: James Donaldson FRCS(Tr & Orth)
Institution: The Royal National Orthopaedic Hospital, Stanmore, London, UK.
Clinicians should seek clarification on whether any implant demonstrated is licensed for use in their own country.
In the USA contact: fda.gov
In the UK contact: gov.uk
In the EU contact: ema.europa.eu



















