Total knee replacement: First stage revision with a hinged spacer and tibial tubercle osteotomy
Overview
Subscribe to get full access to this operation and the extensive Knee Surgery Atlas.
Learn the Total knee replacement: First stage revision with a hinged spacer and tibial tubercle osteotomy surgical technique with step by step instructions on OrthOracle. Our e-learning platform contains high resolution images and a certified CME of the Total knee replacement: First stage revision with a hinged spacer and tibial tubercle osteotomy surgical procedure.
Revision knee surgery for periprosthetic joint infection (PJI) is becoming an increasing burden on the health service. Historically there has been wide variation in surgical practice and this has led to the proposed introduction of knee revision networks, led by the BOA and BASK in conjunction with the NHS. The surgery itself can be challenging and the best results are seen when the surgery is performed by experienced knee surgeons who regularly treat infection.
All patients should be managed within an MDT environment. Experienced orthopaedic surgeons and infectious disease specialists should be present and there should be access to plastic surgeons, pharmacists and rehabilitation teams.
There is no one diagnostic test for PJI and the diagnosis can sometimes be difficult to make. The International Consensus Meeting (Parvizi 2013) define a PJI when:
- There are two positive periprosthetic cultures with phenotypically identical organisms
- There is a sinus tract communicating with the joint
- Three of the following six criteria exist:
- Elevated serum C-reactive protein (CRP) AND erythrocyte sedimentation rate (ESR)
- A single positive culture
- Elevated synovial fluid white blood cell (WBC) count
- ++ change on leukocyte esterase test strip
- Elevated synovial fluid polymorphonuclear neutrophil percentage (PMN%)
- Positive histological analysis of periprosthetic tissue
In my practice there is no role for arthroscopic intervention or a simple washout as a definitive procedure. Its only use is when a patient is septic and it is a temporising measure. A DAIR (debridement and implant retention) procedure is useful in cases of early or acute infection with a well fixed implant – the earlier the better, ideally within 4 weeks. In chronic infections or where the implant is loose revision surgery is needed (if surgery has been decided upon). The choice between single and two stage surgery should be made by the MDT and based on a number of factors. If there is adequate soft tissue coverage, a known sensitive organism and a good host, then single stage revision surgery is appropriate. In cases with resistant or multiple organisms, fungal infection, soft tissue defects that preclude primary closure, an immunocompromised host or where previous revision surgery has failed, a two stage procedure is indicated. The concurrent antibiotic choice and duration should be determined by the MDT and discussed with the patient.
The patient described in this case had deteriorating pain and function with positive cultures and a raised CRP. The decision for a further two stage procedure was straight forward as she had a resistant coagulase negative Staphylococcus, previously had a failed two stage revision for infection and was unwilling to accept the idea of an amputation.
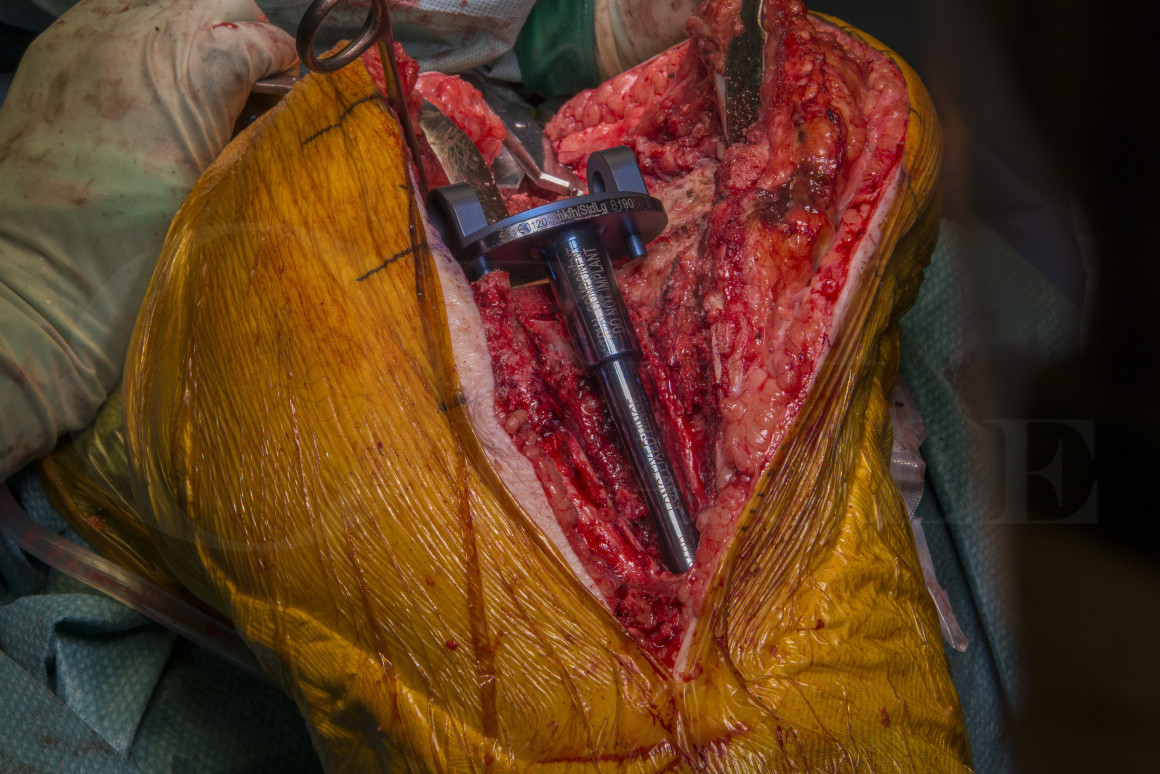
The case uses the Stryker Stanmore Implants Worldwide (SIW) short distal femoral replacement with a fixed hinge. The implant is very easy to use, requires only a few trays with minimal instrumentation and it is fully modular in case the intra-operative plan changes. There are two sizes of knee – standard and small. The tibia comes with either a short 140mm or long 180mm stem with both sizes. A fixed hinge, metal backed rotating hinge and polyethylene rotating hinge are available. 5mm is the minimum resection for the fixed hinge, 8mm for the polyethylene rotating hinge and 11mm for the metal backed rotating hinge. Augments are available from 5 to 20mm for both the tibia and femur.
The fixed hinge is not commonly used. I use it here simply as an articulating spacer as it is cheaper than the rotating hinge version. At the second stage a rotating hinge version will be used.
OrthOracle readers will also find the following operative techniques of interest:
Revision total Knee Replacement- Legion Rotating Hinge Knee ( Smith and Nephew)
Revision total Knee Replacement: Legion CCK (Smith and Nephew)
Author: James Donaldson FRCS (Tr & Orth)
Institution: The Royal National Orthopaedic Hospital, Stanmore, London, UK.
Clinicians should seek clarification on whether any implant demonstrated is licensed for use in their own country.
In the USA contact: fda.gov
In the UK contact: gov.uk
In the EU contact: ema.europa.eu
Online learning is only available to subscribers.



















